| Cancer Type |
Pan-Cancer |
Pan-Cancer |
Pan-Cancer |
| Content Specifications |
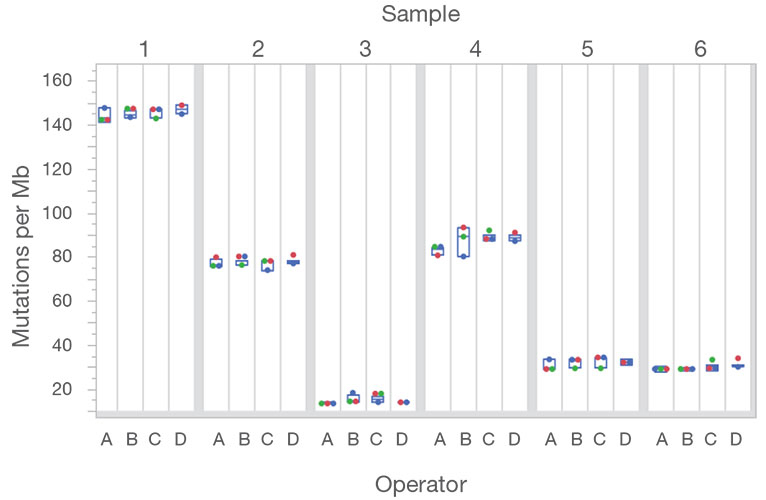
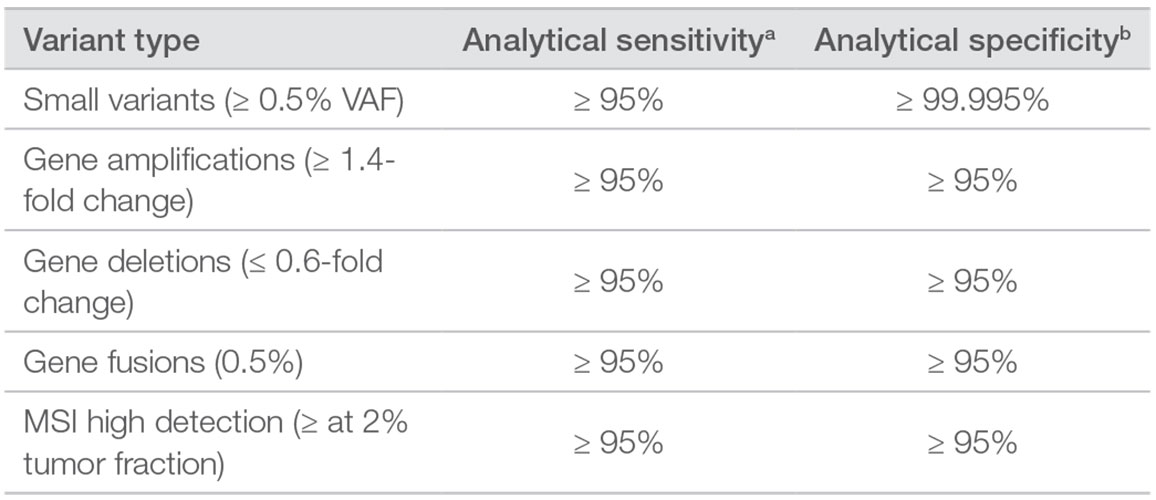
Targeted selection of 523 genes (full coding sequence) for a total of 1.94Mb panel size.
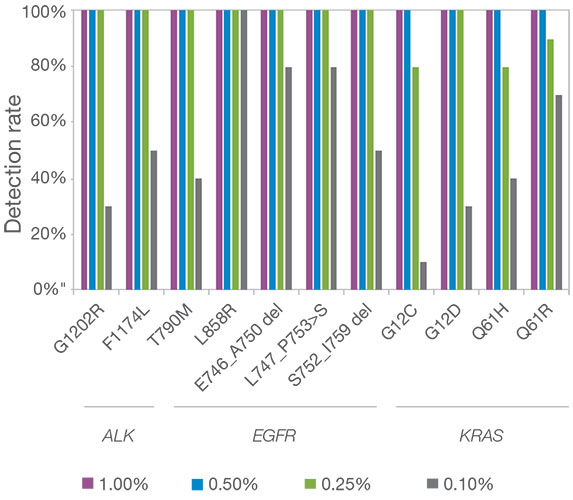
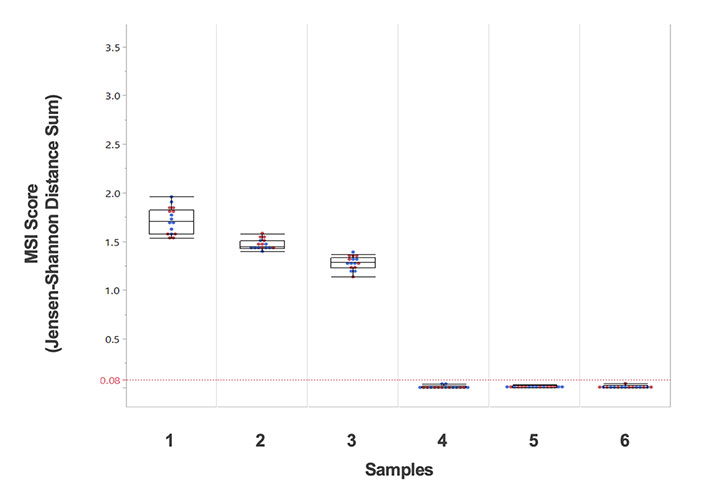
• Immuno-oncology Biomarker Coverage: TMB and MSI
• Guideline Coverage: Broad coverage of key guidelines for multiple solid tumor types
• Clinical Trials Coverage: Over 600 clinical trials* |
Targeted selection of DNA from 523 genes of interest, and RNA from 55 genes, for a total of 1.94Mb panel size.
• Guideline Coverage: Broad coverage of key guidelines for multiple solid tumor types
• Clinical Trials Coverage: Over 1,000 clinical trials
• Immuno-oncology Biomarker Coverage: Biomarkers TMB and MSI included; also inclusive of HLA regions, POLE1 and POLD1*
• TruSight Oncology 500 HRD** kit content includes coverage of ~25,000 SNP's to assess homologous recombination deficiency through a comprehensive genomic instability score (LOH+TAI+LST), powered by Myriad Genetics
• TruSight Oncology 500 HRD** is an optional add-on kit to TruSight Oncology 500
**Not available in Japan |
Targeted selection of DNA from 523 genes of interest, and RNA from 55 genes, for a total of 1.94Mb panel size.
• Immuno-oncology Biomarker Coverage: TMB and MSI
• Guideline Coverage: Broad coverage of key guidelines for multiple solid tumor types
• Clinical Trials Coverage: Over 600 clinical trials* |
| Hands-On Time |
Manual: ~10.5 hrs
Automated: N/A |
Manual: ~10.5 hrs
Automated: ~2.5 hrs (TruSight Oncology 500 only) |
Manual: ~10.5 hrs
Automated: ~2.5 hrs |
| Input Quantity |
30 ng cfDNA (8-10 ml of plasma) |
40 ng DNA, 40 ng RNA |
40 ng DNA, 40-80 ng RNA (need at least 2 mm3 FFPE tissue) |
| Method |
Target Enrichment, Target Enrichment, Targeted DNA Sequencing |
Target Enrichment, Target Enrichment, Targeted DNA Sequencing, Targeted RNA Sequencing |
Target Enrichment, Target Enrichment, Targeted DNA Sequencing, Targeted RNA Sequencing |
| Nucleic Acid Type |
DNA |
RNA, DNA |
RNA, DNA |
| Specialized Sample Types |
Blood, Circulating Tumor DNA |
FFPE Tissue |
FFPE Tissue |
| Species Category |
Human |
Human |
Human |
| System Compatibility |
NovaSeq 6000, NovaSeq 6000Dx in Research Mode |
NextSeq 500, NextSeq 550, NextSeq 550Dx in Research Mode |
NovaSeq 6000, NovaSeq 6000Dx in Research Mode |
| Technology |
Sequencing |
Sequencing |
Sequencing |
| Variant Class |
Copy Number Variants (CNVs), Gene Fusions, Insertions-Deletions (indels), Single Nucleotide Variants (SNVs), Somatic Variants |
Copy Number Variants (CNVs), Gene Fusions, Insertions-Deletions (indels), Single Nucleotide Variants (SNVs), Transcript Variants |
Copy Number Variants (CNVs), Gene Fusions, Insertions-Deletions (indels), Single Nucleotide Variants (SNVs), Transcript Variants |